Medical Device
Regulatory Solutions™
Global Regulatory, Product Development & Clinical Research Consultants

We provide translational development support to biotechnology companies
MDRS, LLC is an FDA registered company that specializes in providing strategic FDA and global regulatory, product development, quality and clinical research support to medical device and biotechnology companies and their development teams.
While MDRS specializes in providing global regulatory support to emergent medical device companies, the firm’s clientele include Fortune 500 biotechnology companies and leading Academic Medical Centers that have utilized the firm’s regulatory, clinical research and quality systems implementation and remediation services.
MDRS, LLC is a Founding Member of the NIH MPW REGENERATIVE MEDICINE RESOURCE CENTER: 2017-2025
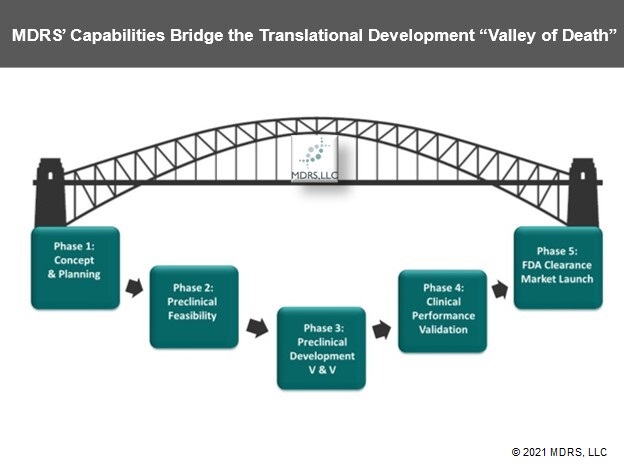
Translational Development Services
MDRS provides comprehensive Total Product Life Cycle (TPLC) translational development consulting services to assist you in developing and maintaining high quality medical products for global markets. We help development teams navigate complex global regulatory requirements and share best practice methodologies associated with successful medical products development and commercialization. We provide regulatory, product development, quality and clinical research solutions at reasonable rates, so your team can afford early regulatory expertise to help your company develop high quality products with optimal efficiencies for long term success.

Regulatory Affairs Support

Device Design & Development

Preclinical Support
Clinical Research Support

Quality Management Support
Commercialization Support
Our numbers speak for themselves
Clients Served
years collective biotech development experience
Global Regulatory Submission & Marketing Authorizations
About
Our consulting team includes regulatory, product development, clinical, quality systems and commercialization experts with more than 170 years of combined experience developing and commercializing medical device, pharmaceutical and combination products.
MDRS LEADERSHIP TEAM

Kay Fuller, RAC
SVP, Regulatory & Clinical Research Emeritus

Steve White, Ph.D., RAC
Interim President &
SVP, Product Development

Jennifer Forbes, B.S.E., M.S.
VP, Quality Engineering

Curt Wichern, B.S.I.T., RMSC
Sr. Principal Consultant – Software Engineering

Lyne Charron-Keller, R.N.
Sr. Principal Consultant – Commercialization
Biotech Product Experience

Cardiovascular Devices
- ePFTE Vascular Grafts, Sutures and Patches
- Vena Cava Filters
- Pacemakers / ICDs
- Left Ventricular Assist Devices (LVADs)
- AAA Endovascular Grafts
- Coronary Artery Imaging Devices (OFDI / OCT / IVUS)
- Biliary and Drug Eluting Stents
- AF Diagnostic & Ablation Systems
- Peripheral Intravascular (PIV) Devices
- CABG, CPB Pumps & ECMO Devices
NeuroSurgical Devices
- Cerebral Spinal Fluid (CSF) Brain Shunts
- Ventricular Access Ports
- CSF Monitoring
- Morphine Drug Delivery Devices – Intrathecal Delivery – Combination Products
- Cavatron Ultrasonic Surgical Aspirators
- Surgical Microscopes
- Cranial Orthosis
- Deep Brain Stimulators (DBS)
- MRI Systems & MR Imaging Software Applications

Additional Product Categories
- MRI Imaging / Post-Acquisition Software
- Combination Products – Drug/Biologic Devices
- Non-Viral Vector Gene Therapy
- Endoscopy, Laparoscopy & Arthroscopy Devices & Imaging Systems
- In Vitro Diagnostic Systems: Molecular Diagnostics (PCR, NGS) & POC
- Clinical Decision Support apps
- Smart Phone apps (Mobile Medical apps)
- Optical Imaging Devices in Multimodal Platforms
- Orthopedic Implants & Prosthetic Ligaments
- Robotic Surgical Systems & Orthotics
- Adjustable Gastric Banding Systems
- Biologic and Pharmaceutical Oncology Drugs & Delivery Devices
clients


























CLIENTS (Partial List)
Biotech Companies
Abbott IVD
Airway Innovations
Alerje
Arbor Medical Innovations
Bodymatter
Cohera Medical
Danmar Products
Tangent Medical
The Patient Company
NeuroNexus
Navigate Surgical Technologies
NeuMoDx Molecular – Qiagen
Histosonics
Leica
Hygieia
Mar-Med
Microdermics
Navigate Surgical
Pixel / Ultrasound Medical Devices
IntroMedic
SpinTech
Sharp Laboratories
St. Blaise
Stemplant
Terran Biosciences
Academic Medical Centers
University of Michigan
Stanford University
Grand Valley State University
University of Pittsburgh – McGowan Institute for Regenerative Medicine
University of Washington
University of Louisville
University of California
Cleveland Clinic
Covenant Healthcare
Magee-Womens Research Institute & Foundation
Michigan State University
Norton Healthcare
Oregon Health Science University
Tufts University
Wayne State University
Washington University in St. Louis
University of Texas
Vanderbilt University
Wyss Institute – Harvard University
Academic Alliances
News & Publications
News
MDRS, LLC is a Founding Member of the NIH MPW REGENERATIVE MEDICINE RESOURCE CENTER: 2017-2025
MDRS, LLC Collaborator on NIH $2.3M Fast-Track Award for Translational Development of Fetal Hydrocephalus Shunt
Arbor Medical Innovations Receives FDA 510(k) Clearance for VERABAND™
Terran Biosciences Receives FDA Clearance for NM-101, the industry’s first software for the analysis of neuromelanin-sensitive MRI | Business Wire
SpinTech Receives FDA 510(k) Clearance for STAGE Technology
NeuMoDx Molecular Gains FDA 510(k) Clearance for NeuMoD 288 Molecular System & NeuMoDx GBS Assay
SpinTech announces 510k clearance of SPIN-SWI - SpinTech MRI
Selected Publications
Taylor DP, Yoshida M, Fuller K, Giannobile WV, Sfeir CS, Wagner WR, Kohn DH. Translating Dental, Oral, and Craniofacial Regenerative Medicine Innovations to the Clinic through Interdisciplinary Commercial Translation Architecture. J Dent Res. Apr 27, 2021
Baman, TS, et al., Pacemaker Reutilization: An Initiative to Alleviate Symptomatic Burden Associated with Bradyarrhythmia in Impoverished Nations Around the World, AHA Circulation, October 19, 2010.
Support for Investigator-Initiated Clinical Research: The Clinical Translational Science Award (CTSA) Experience, Academic Medicine: February 2011 – Volume 86 – Issue 2 – pp 217-223; NIH-CTSA National IND/IDE Taskforce, co-author
Kaigler, D, Fuller, KL, Giannobile, WV, Regulatory Process for the Evaluation of Dental Drugs, Devices and Biologics In: Giannobile, WV, Burt, B, Genco, R. ed, Clinical Research in Oral Health, Wiley-Blackwell, released March 2010
National Cancer Institute: Network For Translational Research (NTR) Optical Imaging In Multimodal Platforms (U54): Year 1 Report: RFA-CA-08-002
Contact US
Company Headquarters
MDRS, LLC
230 Collingwood Dr., Suite 260
Ann Arbor, MI 48103
Grand Rapids Satellite Office:
Doug Meijer Medical Innovation Building
Grand Rapids, MI 49503
Hours
Mon – Fri: 9AM – 5PM, EST
Sat & Sun: Closed
After Hours by Appointment Only
© 2010 – 2025 MDRS, LLC | Built by Buddy Web Design & Development